Al C2h3o2 3 Compound Name
What is Acetate?
Acetate is a chemical compound with formula C2H3O2 −. It is also known as Acetate Ion or Monoacetate. Information technology is a salt formed by the combination of acetic acid with alkaline metal, metal, earthy, nonmetallic or other bases.
Tabular array of Contents
- Structure Of Acetate
- Fermentation of Acetate
- Properties of Acetate
- Chemic Properties of Acetate
- Uses Of Acetate
- Frequently Asked Questions – FAQs
The conversion of pyruvate to acetyl-coenzyme A is carried out with the help of an enzyme called pyruvate dehydrogenase. The acetyl-CoA is farther converted to acetate in E. coli and simultaneously produces ATP by substrate-level phosphorylation. The entire process requires 2 enzymes viz acetate kinase and phosphate acetyltransferase.
acetyl-CoA + Phospate → acetyl-Phospate+CoA
acetyl-Phospate+ADP → acetate + ATP
Structure Of Acetate (C2H3O2 −)
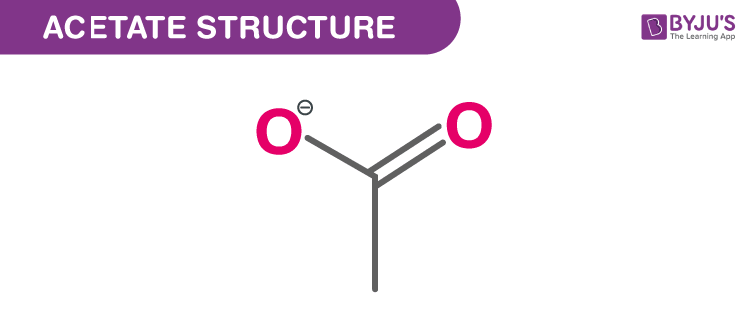
Fermentation of Acetate
Acetic acid undergoes dismutation reaction to generate carbon dioxide and methane.
CHthreeCOO− + H+ → CH4 + CO2 ΔM° = −36 kJ/mol
The reaction is carried out in the presence of catalyst methanogen archaea. One electron is transferred from the carboxylic grouping to the methyl group of acetic acrid to generate carbon dioxide and methane gas.
Properties of Acetate (C2H3O2 −)
| Acetate | C2H3Oii − |
| Molecular Weight of Acetate | 59.044 chiliad/mol |
| Monoisotopic Mass of Acetate | 59.013 m/mol |
| Complexity | 25.v |
| Conjugate acid | Acerb acid |
Chemic Backdrop of Acetate – (C2H3O2−)
1. Acetate reacts with sodium hydroxide
Sodium acetate and soda lime(NaOH + CaO), on reaction give marsh gas and sodium carbonate. This reaction is called decarboxylation and is one of the general methods to prepare alkanes.
CHthreeCOONa + NaOH → CH4 + NaiiCO3
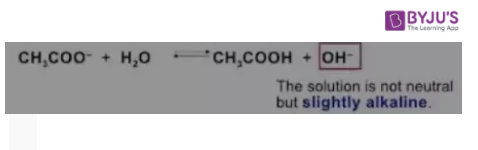
2. Acetate reacts with Water
H2o spontaneously ionizes into hydroxide anions and hydrogen cations. Sodium acetate dissociates in water into sodium and acetate ions. Sodium ions react very little with the hydroxide ions whereas the acetate ions combine with hydrogen ions to produce acetic acid.
Uses Of Acetate (C2HthreeO2 −)
-
- Acetate is used as a solvent in paints, inks, coatings.
- Cellulose acetate is used eyeglass frame.
- It is used in diapers.
- Potassium acetate is used every bit a food preservative.
- It is used in laboratories.
- Aluminium acetate is used as an anti stringent.
Frequently Asked Questions – FAQs
What elements are in acetate?
Both metallic acetates are inorganic salts of a metal cation and acetate anion, a polyatomic ion consisting of two carbon atoms ionically jump to three hydrogen atoms and two oxygen atoms (Symbol: CH3COO) with a full formula weight of 59.05.
What is the difference betwixt acetate and acetic acrid?
The main stardom between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a cyberspace negative electrical charge. Acetic acid is an organic chemical compound that helps create vinegar while acetate ion is the acetic acid's conjugate base of operations.
Is Acetate a base or acrid?
Sodium acetate is a basic salt; the acetate ion will deprotonate h2o, thereby increasing the pH of the solution. The basic salt are formed in the neutralization reaction between a strong base and a weak acrid.
What are the advantages of acetate?
This is among the most flexible fabrics and can resist wrinkling. ADVANTAGES: Has a silky advent, a luxurious feel to information technology. DISADVANTAGES: The dyes can fade or bleed, are decumbent to rut, and are relatively poor fibres. Paw wash acetate clothes with warm water, and just a low-cal-duty detergent.
How is acetate produced?
The acetate fiber is developed past using acetic anhydride to react with high purity wood pulp. The acetate flakes formed past this chemical reaction are dissolved in a solvent, filtered, and modified to obtain a solution for spinning stock.
Learn more about the chemical behaviour and importance of acetate (CtwoH3Oii −) with the skillful faculties at BYJU'S.
Al C2h3o2 3 Compound Name,
Source: https://byjus.com/chemistry/acetate/
Posted by: ackermanpubleausing1955.blogspot.com


0 Response to "Al C2h3o2 3 Compound Name"
Post a Comment